Fractured but Alive: the Biology Behind Bone Healing
- golabiromtin
- Apr 18, 2025
- 5 min read
Updated: Jun 23, 2025
By Romtin G.
When I fractured my tibia, the pain wasn’t the only thing that caught me off guard—it was the quiet complexity of what came after. Bones seem so solid, so permanent, that it’s easy to forget they’re made of living, dynamic tissue. But the moment a fracture occurs, biology kicks in with a carefully orchestrated healing process that’s as intricate as any surgical procedure. To really understand how a bone recovers from trauma, we first have to look at what it’s made of, what can go wrong, and how the body repairs itself from the inside out.
Bone Composition: A Living Scaffold
Bone is often mistaken for being an inert support system that just helps with movement. But at the microscopic level, bone is a dynamic and highly vascularized tissue made of two main components: an organic matrix and an inorganic matrix. The organic part is about 30-40% of your bone matrix, and it’s made primarily of type I collagen, a protein that provides tensile strength and flexibility to your bone. It also contains proteoglycans, glycoproteins, and osteocalcin, all of which help with structural support and calcium regulation. The inorganic part (60-70% of the matrix), primarily hydroxyapatite (a crystalline form of calcium phosphate, Ca₁₀(PO₄)₆(OH)₂), provides the rigidity bones need to bear weight. It also stores 99% of the body’s calcium and 85% of its phosphorus.
There are two types of bone tissue: compact (cortical) bone, which is dense and forms the outer shell of most bones, and trabecular (spongy) bone, which is lighter, porous, and found at the ends of long bones and inside vertebrae. This dual structure allows bones to be strong and lightweight so that your bones don’t drag you down, but are strong enough to not get fractured every second!
Within bone are osteocytes (mature bone cells that maintain bone tissue), osteoblasts (which build new bone), and osteoclasts (which break down old or damaged bone). Together, they form the remodeling unit that constantly reshapes bone in response to mechanical forces, injury, and metabolic demands.
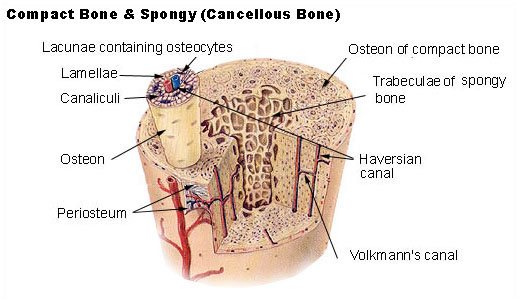
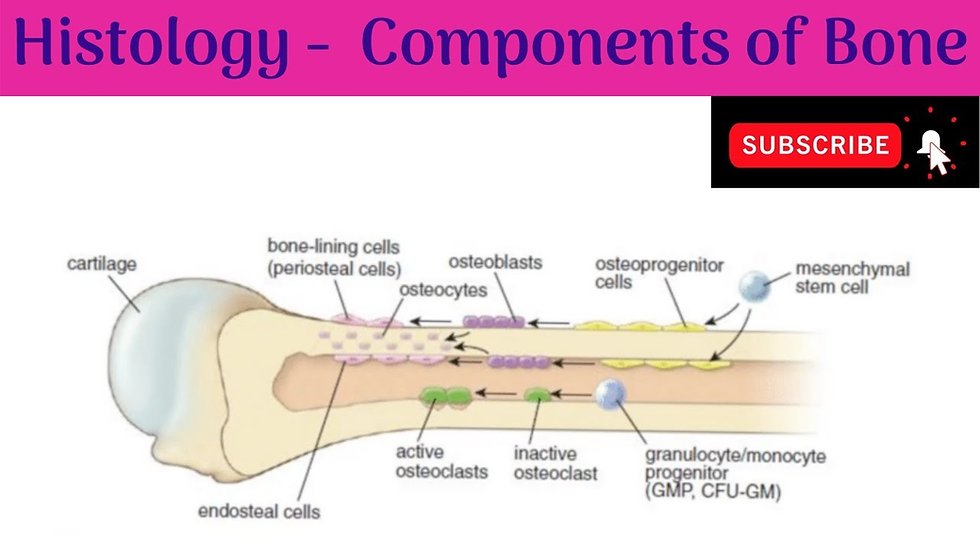
Types of Bone Fractures
Unlike the gigantic hospital gown I had to wear at the hospital, fractures are not one-size-fits-all. Fractures vary in complexity and presentation, often classified based on the pattern of the break, its location, and whether the skin is compromised. A simple fracture involves a clean break without the piercing of the skin, while a compound fracture sees the bone protruding externally, greatly increasing the risk of infection. Understanding the pattern and mechanism of the different kinds can guide treatment and prevention:
Transverse fractures: A straight break across the bone perpendicular to its long axis, often due to direct trauma.
Oblique fractures: Angled breaks that run across the bone shaft
Spiral fractures: Break that winds around the bone’s length due to a twisting force
Comminuted fractures: The bone shatters into three or more pieces, usually seen in high-impact trauma (car crashes).
Greenstick fractures: Incomplete breaks that occur more often in children due to their flexible bones (think about how when you break a green tree branch it splinters rather than breaks).
Stress fractures: Micro-cracks from repeated mechanical loading, especially common in sports that require lots of running and jumping.
Avulsion fractures: Occur when a tendon or ligament pulls off a piece of bone (like my second kneecap dislocation).
Pathological fractures: Happen when bone is weakened by disease, like osteoporosis or cancer.
Each fracture presents a unique challenge to the body’s repair mechanisms and often dictates treatment decisions like casting, bracing, or surgery. Just as much as treatment depends on the break itself, it also hinges A LOT on the person’s age, activity level, and overall bone health. But even if it’s stabilizing with a cast or realigning with surgery, the ultimate goal remains the same: to restore function while minimizing long-term damage, one fracture at a time.
The Four Stages of Bone Healing
While modern medicine can stabilize and align broken bones, it’s the biology underneath that really does the rebuilding and brings you back to 100%. Bone healing is a complicated, multi-phase process, and here’s how it goes:
1. Hematoma Formation (Inflammation Phase): Day 0 to Week 1
The moment a fracture occurs, blood vessels within the periosteum (thin membrane covering bone), cortex, and marrow rupture, leading to localized hemorrhage (bleeding). This forms a hematoma, which is rich in platelets and inflammatory mediators. Platelet degranulation (a process where platelets release contents in their storage granules) releases key factors such as PDGF (Platelet-Derived Growth Factor), TGF-β (Transforming Growth Factor Beta), and VEGF (Vascular Endothelial Growth Factor). These initiate angiogenesis (the development of new blood vessels) and recruit immune cells (neutrophils, monocytes) to the site. Monocytes differentiate into macrophages, which phagocytose (“eat”) necrotic debris and secrete cytokines to sustain the inflammatory response. Importantly, this phase also activates mesenchymal stem cells (MSCs) in the periosteum and endosteum via inflammatory cues. These multipotent cells can differentiate into many types of cells and will drive the next stages of repair.
2. Fibrocartilaginous (Soft) Callus Formation: 1 to 3 Weeks
With inflammation subsiding, activated MSCs differentiate into fibroblasts and chondroblasts, guided by hypoxic conditions (from obstructed blood flow) and the mechanical environment. Fibroblasts lay down a collagen matrix (mainly type I and III) while chondroblasts begin producing a cartilaginous extracellular matrix, rich in type II collagen and aggrecan, to form a fibrocartilage callus. This tissue spans the fracture gap, providing mechanical stability but remaining non-mineralized. It doesn’t have any blood vessels, so it depends on diffusion for nutrient exchange, and it also serves as a template in which mineralized bone will fill in during the later stages.
3. Hard/Bony Callus Formation (Woven Bone Production): 3 to 8 Weeks
Endochondral ossification begins as chondrocytes in the soft callus get larger and mineralize their matrix. These chondrocytes express collagen type X, VEGF (drives vascular invasion), and MMP-13 (degrades cartilage matrix). As capillaries (tiny blood vessels) invade, they bring osteoprogenitor cells that differentiate into osteoblasts, which begin depositing woven bone over the cartilage template. Woven bone is very immature: it’s highly cellular, disorganized, and rapidly laid down. But it’s mineralized and provides rigidity and strength and sets up the final stage of bone repair.
4. Remodeling (Lamellar Bone Formation and Restoration): Months to Years
Over time, the disorganized woven bone is replaced by lamellar bone, which has a highly organized structure and is the normal, mature form of bone in a healthy adult. This remodeling phase is orchestrated by basic units composed of osteoclasts (reabsorb woven bone) and osteoblasts (lay down new lamellar bone in rings around blood vessels). This phase is heavily influenced by the parathyroid hormone (regulates calcium concentrations and osteoclast activity) as well as calcitonin and vitamin D (support mineral deposition).
Bone healing is a brilliant example of orchestrated tissue regeneration, as it’s driven by inflammation, scaffold formation, ossification, and refinement. And despite being super biological, its outcomes are shaped by fracture characteristics, mechanical stability, and systemic conditions (age, nutrition, disease). Understanding the underlying biology helps explain why different fractures heal in different ways and how to approach treatments.

Conclusion
Thanks for reading! I hope you learned something cool about what’s really going on beneath the surface of a fracture!